
Modelling COVID-19 in the middle of the crisis
Published on 30/09/2020

nevil zaveri CC BY 2.0
Back in April, I joined the Evolutionary Ecology of Infectious Disease group in Oxford and the One Health Poultry Hub as a post-doctoral researcher in mathematical modelling. My main project revolves around modelling the networks by which poultry are raised and distributed in India, Bangladesh, Vietnam and Sri Lanka, and the dynamics and evolution of bacterial and viral pathogens along these networks.
When I joined the group, my colleagues at Oxford had recently turned their attention to COVID-19 and in particular to outstanding questions related to COVID-19 immunity. In this context, I was given the opportunity to work with them on multiple projects. One of these projects was about the potential role of cross-reactive responses induced by other endemic human coronaviruses (eHCoVs) on COVID-19 severity.
Challenge of SARS-CoV-2
As an emergent pathogen that recently jumped from an unspecified animal source to humans, SARS-CoV-2 represents a novel challenge to our immune system. Among other things, this means that at the beginning of the pandemic, most of us were susceptible to infection. Widespread population-level susceptibility to a disease is a distinctive feature of most pandemics, and one of the main reasons why it is easy for a novel pathogen to multiply so rapidly across the human population.
However, some individuals that had never been exposed to the virus appeared to ‘recognise’ it to some extent. While the precise role of such pre-existing immunological response is still unknown, its origin is believed to be linked to cross-reactions induced by exposure to other eHCoVs. Currently, four eHCoVs – namely HCOV-229E, -NL63, -OC43 and -HKU1 – are known to co-circulate globally, though they are typically associated with mild respiratory illness. In fact, eHCoVs are among the leading causes behind the common cold.
Eventually, most individuals recover from COVID-19 with little difficulty, sometimes displaying no symptoms whatsoever. Typically, after a few days after infection, the initially naive immune system manages to establish an effective countermeasure against the invading virus.
Some individuals, however, are not as lucky: as the infection progresses, their immune system not only fails to mount an effective response, but also stimulates a systemic inflammation that may extend from the lungs to other organs.
Severity by age
Although only a small proportion of individuals develop severe symptoms, not all strata of the population share the same risk. Rather, the risk of progressing to a severe stage, and eventually requiring intensive care and/or mechanical ventilation, increases rapidly with age. Interestingly, this pattern is in stark contrast with other eHCoVs, where older individuals are typically more resilient to eHCoVs compared to children.
As the reasons behind such age patterns of severity remain unknown, it is imperative to formulate biological hypotheses that might further guide research in the near future.
In my work, we hypothesised that previous exposure to eHCoVs could explain age-specific patterns of COVID-19 severity. Essentially, we posited that as an individual encounters any eHCoV for the first time, he or she becomes temporarily cross-protected against severe symptoms due to COVID-19 infection. At the same time, repeated infections with the same eHCoVs do not add any benefit in terms of cross-protection, but rather weaken the ability of an individual to mount a successful immune response against COVID-19.
In summary:
- As younger individuals first encounter most common viruses (eHCoVs) before they reach adulthood, our hypothetical mechanism predicts the highest level of cross-protection to SARS-CoV-2 among children.
- On the other hand, the elderly are too ‘over-specialised’ to common viruses due to a lifetime of recurrent eHCoV infections, and thus they are less able to develop new responses to SARS-CoV-2 efficiently, and are at increased risk of developing severe symptoms.
Modelling hypotheses
We illustrated our ideas through a modelling approach. As a first step, we implemented an individual-based model of eHCoV co-circulation. Within this framework, we could track individuals’ histories of exposure to eHCoVs as they aged. Based on this information, we assigned to each individual a probability to develop severe symptoms (upon infection with SARS-CoV-2) that depended only on their past history of exposure and according to our hypothesised mechanism. We showed that our mechanism is compatible with the hospitalisation rates observed in different European countries.
As a young infectious disease modeller, working on COVID-19 during these times represented a truly positive experience. I was able to rapidly gain substantial knowledge about a variety of topics, including notions of immunology and eHCoV epidemiology, as well as hone my skills as a modeller, and grow as a scientist overall.
This experience has also made me more aware of the challenges associated with theoretically-oriented research. Indeed, the speculative nature of our work prevented several journals from considering our manuscript for publication. Our hope is that our work could stimulate further research into the drivers behind COVID-19 severity and the nature of cross-reactive responses induced by eHCoVs.
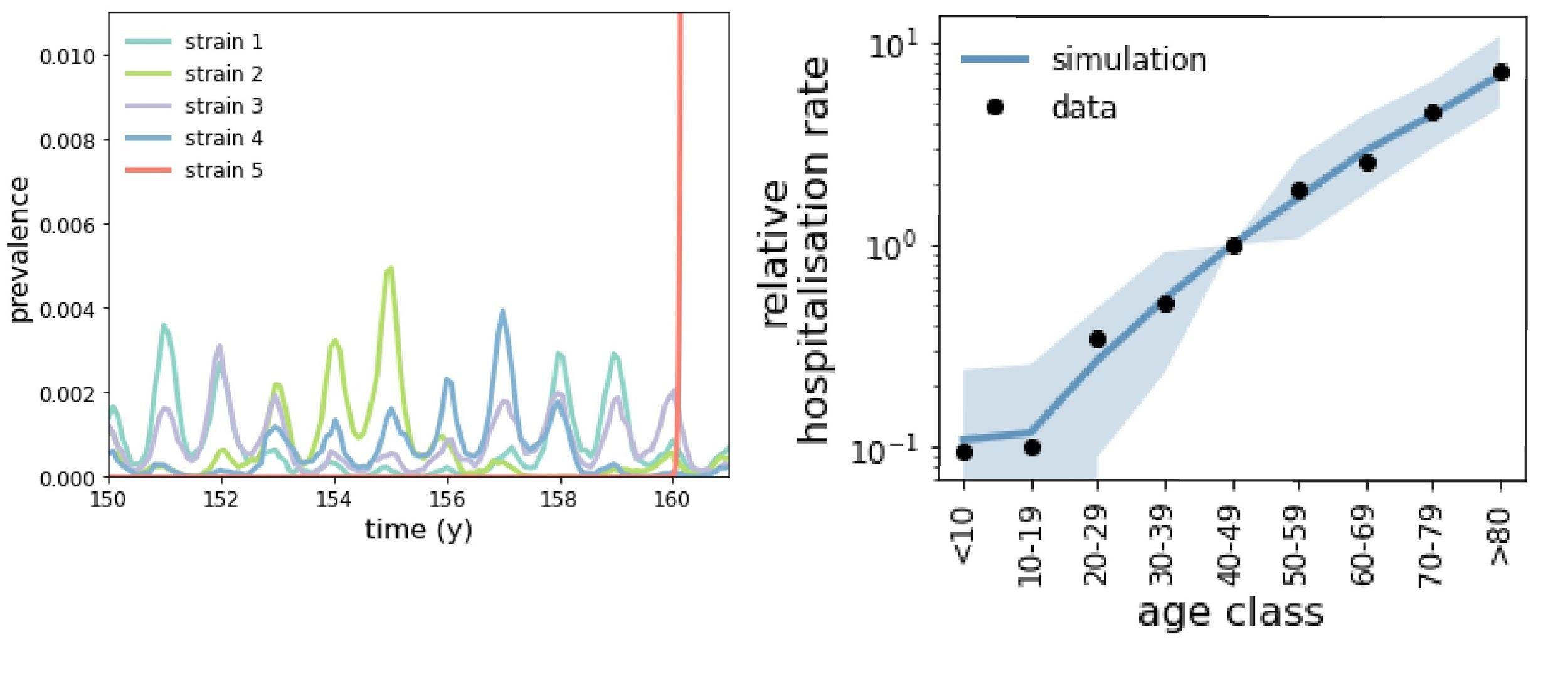 Left: co-circulation dynamics of multiple eHCoVs (strains 1-4) and emergence dynamics of SARS-CoV-2 (strain 5, in red). Right: relative rate of COVID-19 hospitalisation in Europe by age class, as provided by ECDC (dots) and by our fit (blue).
Left: co-circulation dynamics of multiple eHCoVs (strains 1-4) and emergence dynamics of SARS-CoV-2 (strain 5, in red). Right: relative rate of COVID-19 hospitalisation in Europe by age class, as provided by ECDC (dots) and by our fit (blue).


